“ThrombUS+” project funded with € 9.5 million
EU project develops portable diagnostic device for the early detection of deep vein thrombosis
Deep vein thrombosis (DVT) is a significant health risk. In about half of patients, the blood clot breaks away from the vein wall and travels to the lungs, where it can cause a pulmonary embolism. Approximately 25% of people who suffer a pulmonary embolism die as a result. This makes it the third leading cause of cardiovascular death worldwide, after stroke and heart attack. The danger: In up to two-thirds of all cases of thrombosis, people have no symptoms. This makes early detection a major challenge. The EU project "ThrombUS+" brings together 18 European partners to develop a new, portable diagnostic device. The EU is funding the project with €9.5 million as part of the Horizon Europe Innovation Action.

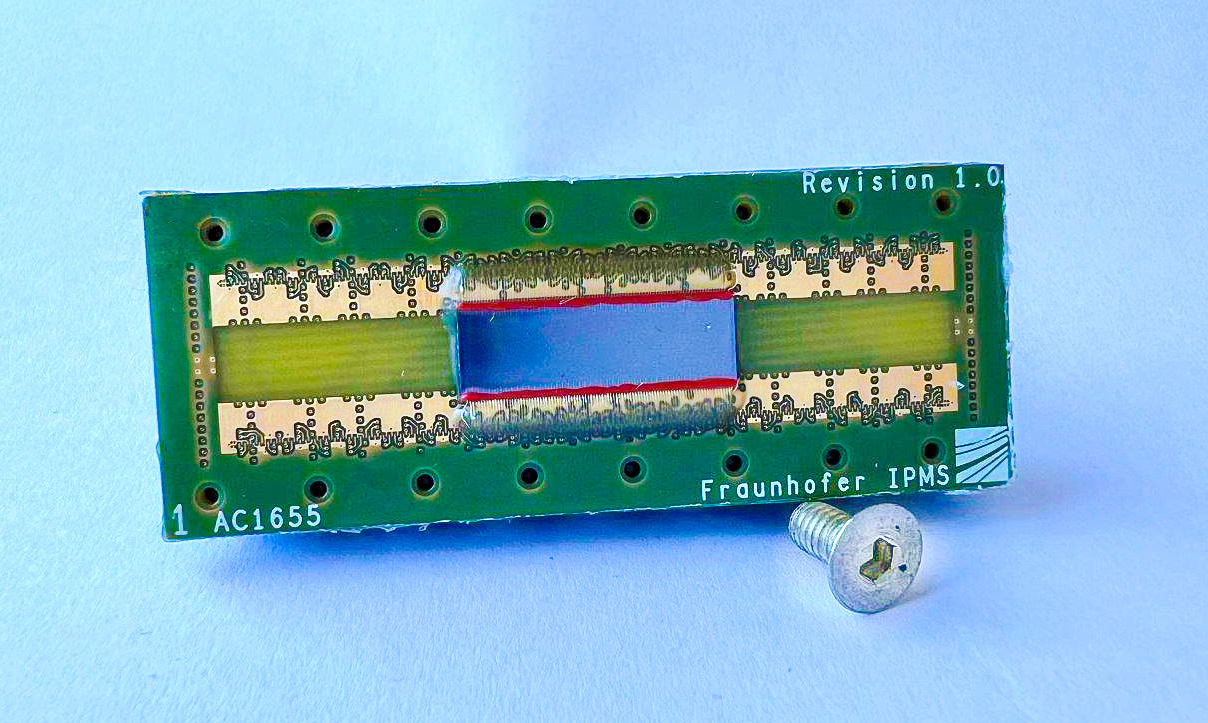
The ThrombUS+ project plans a portable cuff with an integrated ultrasound transducer for continuous vascular imaging of the lower limbs to detect venous thrombosis as soon as it occurs. In the project, Fraunhofer IPMS and VERMON are developing ultrasound transducer arrays for the portable component, which will enable continuous monitoring of DVT directly on site. Fraunhofer IPMS focuses on MEMS-based ultrasound transducers, so-called CMUTs (Capacitive Micromachined Ultrasonic Transducers). They are considered to be the next generation of medical ultrasound sensors. The low-cost mass production of CMUTs makes them widely available. Their advantages, such as miniaturization with a high number of channels, high bandwidth and sensitivity, open up the possibility of developing a completely new diagnostic system.
"There are many challenges in developing a portable solution for the diagnosis and prevention of thrombosis," said Prof. Kaldoudi, coordinator of the project and a scientist at the Athena Research Center in Greece. "We are addressing these challenges not only from a technological perspective. One of the strengths of the consortium lies in the application of a compliance-by-design approach, which integrates legal, regulatory and safety requirements for complex medical devices as early as possible in the development process, thus shortening the innovation's path to the patient".
The clinical, technical and regulatory experience gained in the ThrombUS+ project will therefore create new opportunities for market access of future complex AI-based medical devices. The project paves the way for a new era in which wearable devices and artificial intelligence can be used to transform diagnostics into continuous and autonomous point-of-care services. This will reduce the burden on physicians. The interdisciplinary and international composition of the consortium is crucial to its success.
 Fraunhofer Institute for Photonic Microsystems
Fraunhofer Institute for Photonic Microsystems